Allylic Vs Benzylic Or Vinylic
I have heard about allylic vinylic benzylic carbons but positions.
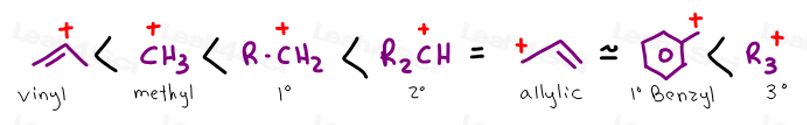
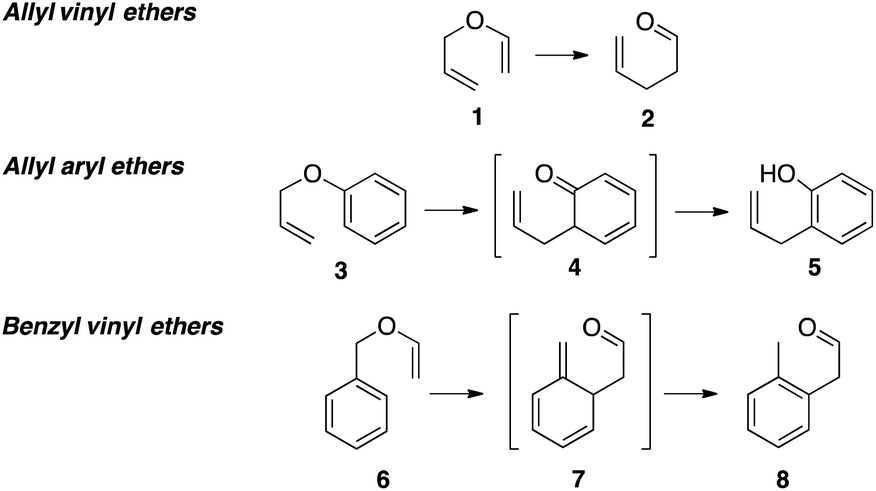
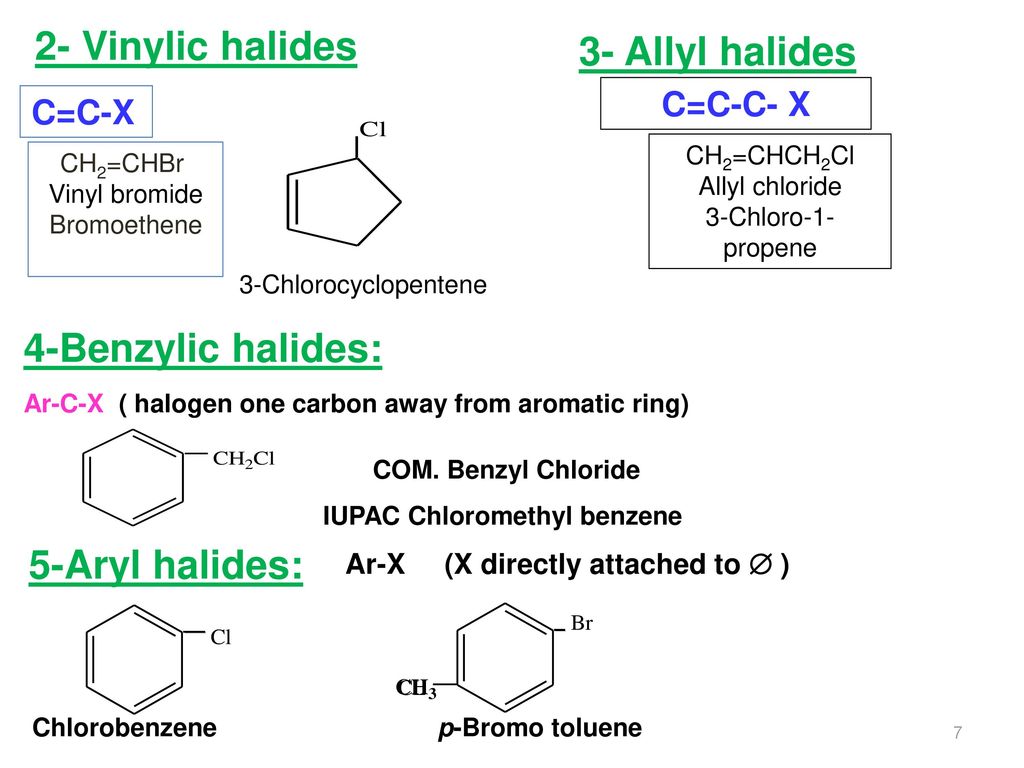
Allylic vs benzylic or vinylic. Would x in c c x be vinylic on the same train of thought claisen rearrangements utilize vinylic allyl ethers. Allyl group holds three carbon atoms and five hydrogen atoms. Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule. Carbo cations may be stabilized by.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation. Allyl h 2 c chch 2 rapid s n 2 substitution for 1º and 2º halides. But would the c bound to the 2 carbons in a double bond also be considered an allylic carbon. An allylic hydrogen is a hydrogen atom that is bonded to an allylic carbon in an organic molecule.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds. For 3º halides a very slow s n 2 substitution or if the nucleophile is moderately basic e2 elimination. Vinyl group vinylic hydrogen vinylic carbocation allylic position benzylic position propargylic position wikipedia entry return to glossary index. Vinyl group has two carbon atoms and three hydrogen atoms.
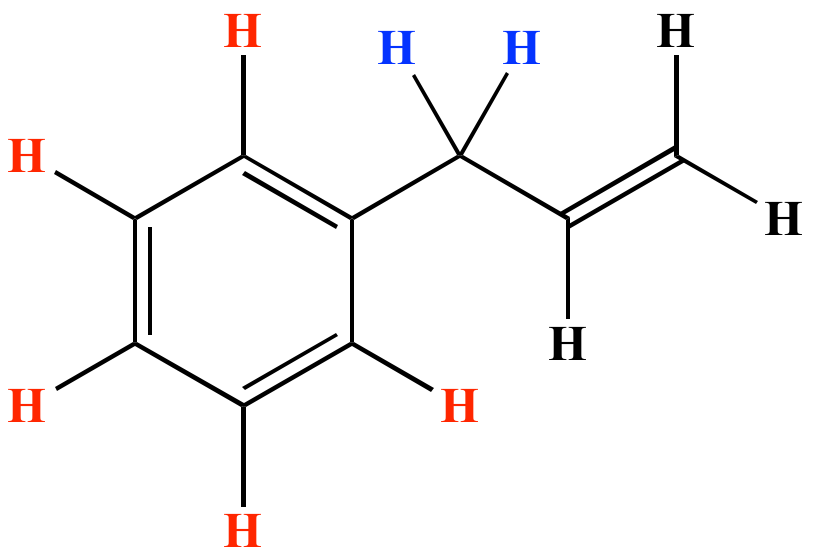
In high dielectric ionizing solvents such as water dimethyl sulfoxide acetonitrile s n 1 and e1 products may be observed. None of the other hydrogens are vinylic. Benzene c x where x is the benzylic group vinylic. The vinylic hydrogens are shown in red.
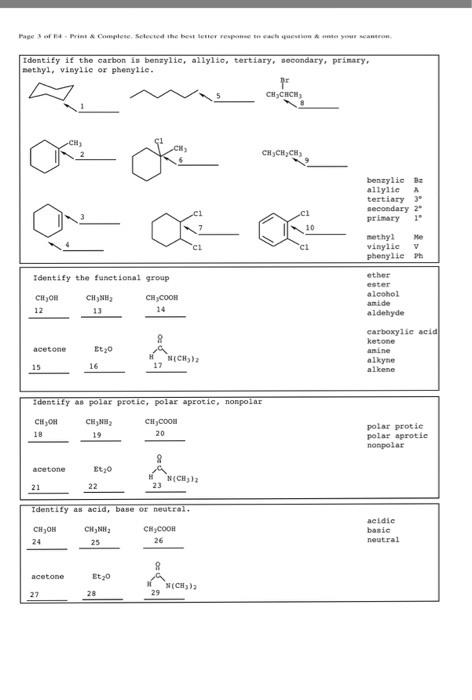
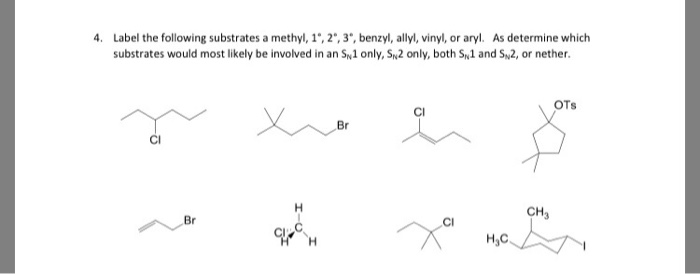
So x in c c c x would be an allylic group. Vinylic carbocations are unstable as they lack p character. Allyl group gets attached to any other group of atoms through. In this video we will learn everything about how we can identify allylic vinylic benzylic pehnylic carbon and how to name the compounds related to those.
Allyl groups have three carbon atoms and five hydrogen atoms. Benzylic position allylic position propargylic position aryl aryl hydrogen methyl hydrogen primary hydrogen secondary hydrogen tertiary. Rapid s n 2 substitution for 1º. Allyl form a stable carbocation because of the electron delocalization.