Allylic Halide Vs Vinyl Halide
Identify allyl vinyl phenyl benzyl groups or substituents name their compounds cbse jee neet.
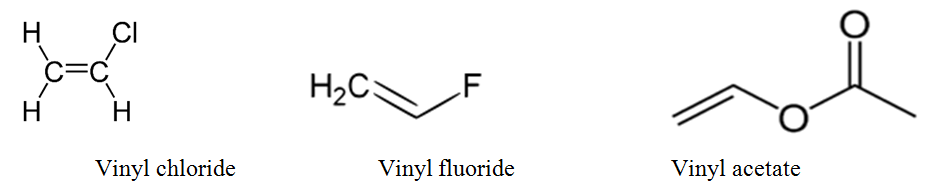
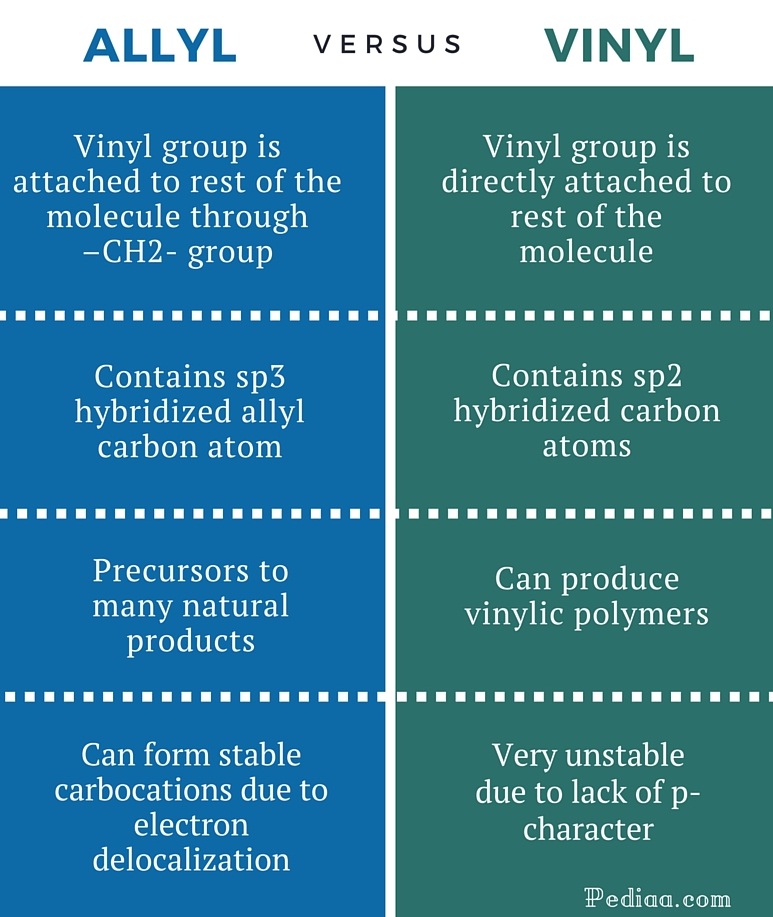
Allylic halide vs vinyl halide. Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation. Allyl h 2 c chch 2 rapid s n 2 substitution for 1º and 2º halides. Rapid s n 2 substitution for 1º. A vinyl halide is clearly a species with a formula h 2c c x h in which a halide is directly bound to an olefinic bond.
They are subdivided into alkyl vinylic aryl and acyl halides. However alkyl halides may sometimes be confused with aryl halides. In alkyl halides all four bonds to the carbon that bears the halogen are single bonds. S n 2 reactions of allylic halides and tosylates.
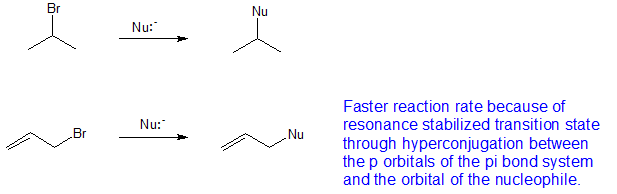
They exhibit faster s n 2 reactivity than secondary alkyl halides because the bimolecular transition state is stabilized by hyperconjugation between the orbital of the nucleophile and the conjugated pi bond of the allylic. Why aryl halides are less reactive than alkyl halides for nucleophilic substitution. An allylic halide is an alkyl halide in whose molecule there are one or more halogen atoms on allylic carbons. Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
The key difference between these two structural components is the number of carbon and hydrogen atoms. In high dielectric ionizing solvents such as water dimethyl sulfoxide acetonitrile s n 1 and e1 products may be observed. Allylic halides and tosylates are excellent electrophiles for bimolecular nucleophilic substitution reactions s n 2. For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides.
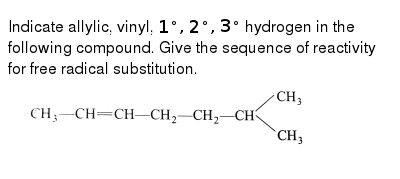
Halogens are more electronegative than carbon. An allyl group is a substituent with the structural formula h 2 c ch ch 2 r where r is the rest of the molecule. The name is derived from the latin word for garlic allium sativum in 1844 theodor wertheim isolated an allyl derivative from garlic oil and named it schwefelallyl. Allyl groups have three carbon atoms and five hydrogen atoms.
Vinyl chloride h 2c chcl is an example. Other articles where vinylic halide is discussed. For 3º halides a very slow s n 2 substitution or if the nucleophile is moderately basic e2 elimination. In aryl halides the halogen bearing carbon is part of.
For example if the halogen atom is attached to a carbon atom which is attached to a benzene ring cl ch 2 c 6 h 5 one would think it is an aryl halide but it is an alkyl halide because the halogen atom is attached to the carbon that is sp 3 hybridized. Formally this is ethylene h 2c ch 2 with one of the hydrogens substituted by a heteroatom. A vinylic halide from an aryl halide. In organic chemistry a vinyl halide is a compound with the formula ch 2 chx x halide the term vinyl is often used to describe any alkenyl group.
An aryl halide has general formula c 6h 5x in which an halide group x has substituted the aryl ring.