Allyl Chloride And Vinyl Chloride

Allyl ligands bind to metallic centers through its three carbon atoms.
Allyl chloride and vinyl chloride. Vinyl chloride is used primarily to make polyvinyl chloride pvc. The carbon atoms in the chemical structure of allyl chloride are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated each carbon atom is considered to be associated with enough hydrogen atoms to provide the. Allyl chloride was added to a previously prepared suspension of aluminium chloride in phosphorus trichloride molar ratios. 0 5 and the mixture was stirred for 30 min at 40 50.
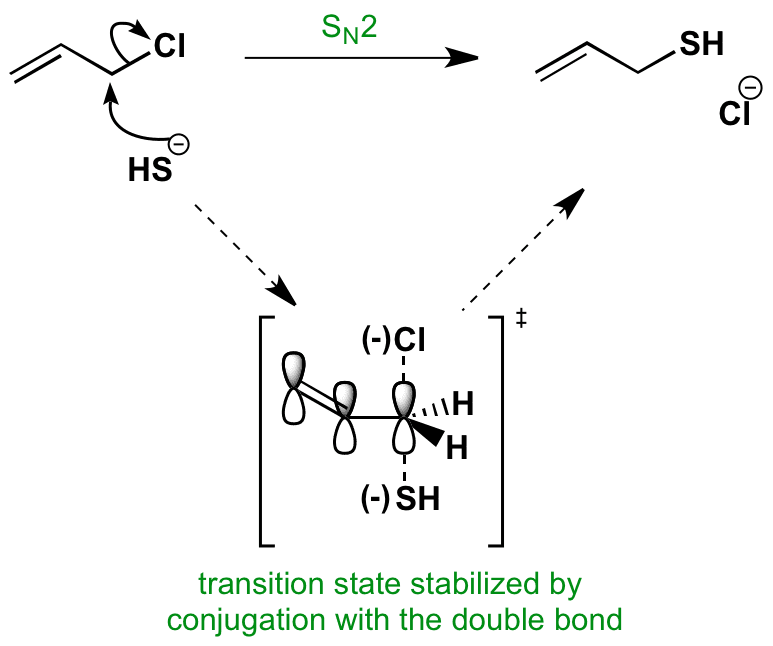
See first sn1 and sn2 is substitution and it s preferred nucleophilic substitution so. 1 0. Most of vinyl derivatives are used in polymer industry. Hence the bond pair of electrons of c cl bond in allyl chloride are less strongly held than that in vinyl chloride and the removal of chlorine is easier in allyl chloride than in vinyl chloride.
Exposure to allyl chloride primarily occurs for workers in manufacturing plants. Vinyl chloride is a colorless gas that burns easily. Ch 2 chch 2 cl. It does not occur naturally and must be produced industrially for its commercial uses.
S p 2 carbon atom is more electronegative than s p 3 carbon atom. The acute short term effects of allyl chloride from inhalation exposure in humans consists of irritation of the eyes and respiratory passages. Allyl chloride cas number 107 05 1 appearance clear liquid assay 99 0 min. Vinyl chloride vinyl fluoride vinyl acetate vinylidene and vinylene.
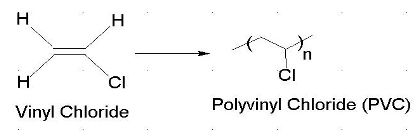
About 13 billion kilograms are produced annually. Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc. What is vinyl chloride. Log oil water 0 2 solubiliy.
Allyl compounds are of a wide range and are used in several areas. Specific gravity 0 94 water 1 odour pungent. Alcl 3 0 25. After this the complex which had formed was diluted with 5 10 times its volume of methylene dichloride and the mixture obtained was cooled to 20 by.
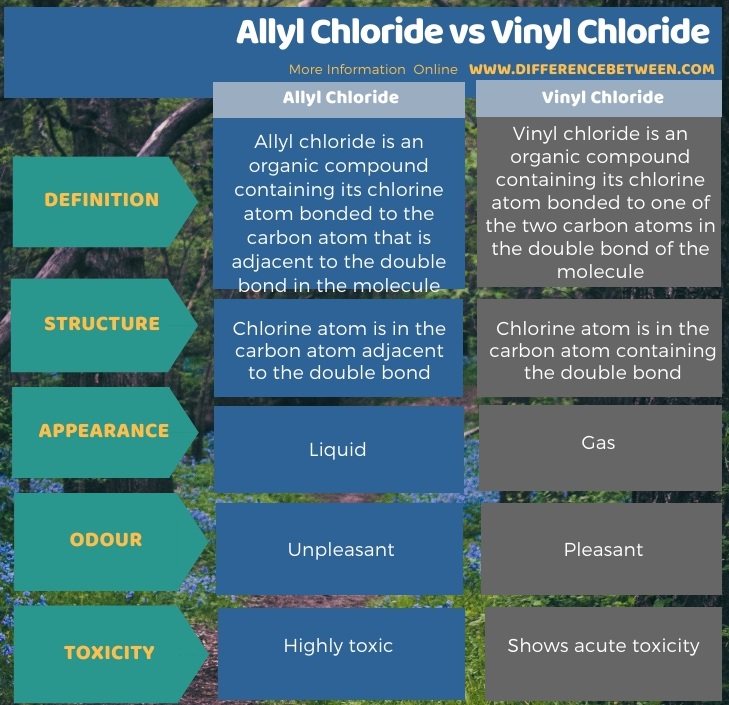
Chronic long term exposure to allyl chloride in humans causes injury to the liver and kidneys and the onset of. The key difference between allyl chloride and vinyl chloride is that ally chloride contains its chlorine atom bonded to the carbon atom that is adjacent to the double bond whereas vinyl chloride contains its chlorine atom bonded to one of the two carbon atoms in the double bond. The 2d chemical structure image of allyl chloride is also called skeletal formula which is the standard notation for organic molecules. So you see that sn1 mechanism involving in carbocation intermediate both allylic and benzylic halide having resonance so it s increase its stability of there.
In vinyl chloride chlorine is attached to s p 2 carbon atom.