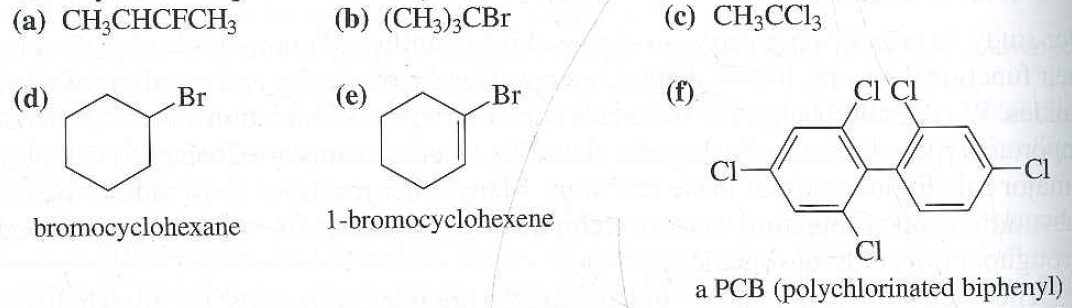
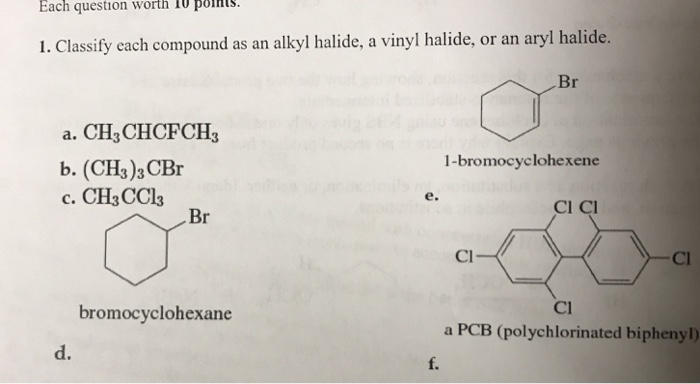
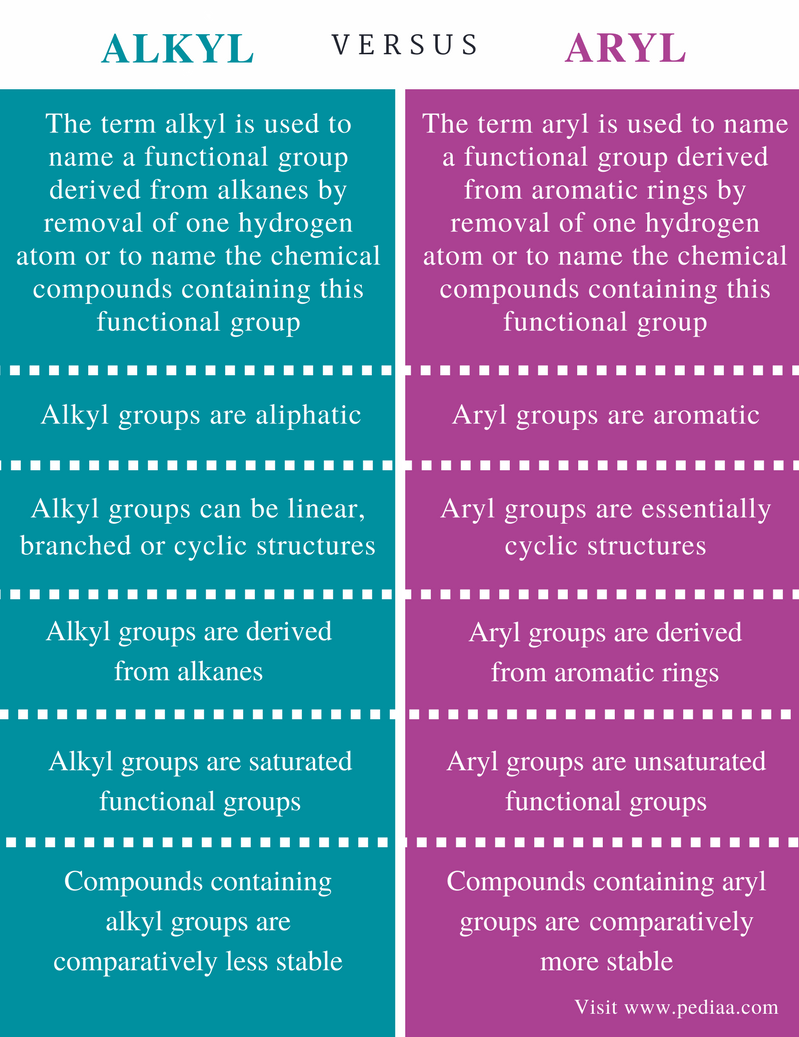
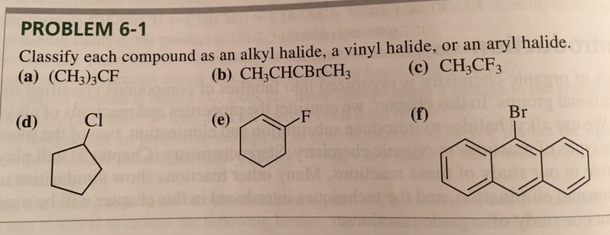Alkyl Vinyl Aryl Halides
With the exception of iodine these halogens have electronegativities significantly greater than carbon.
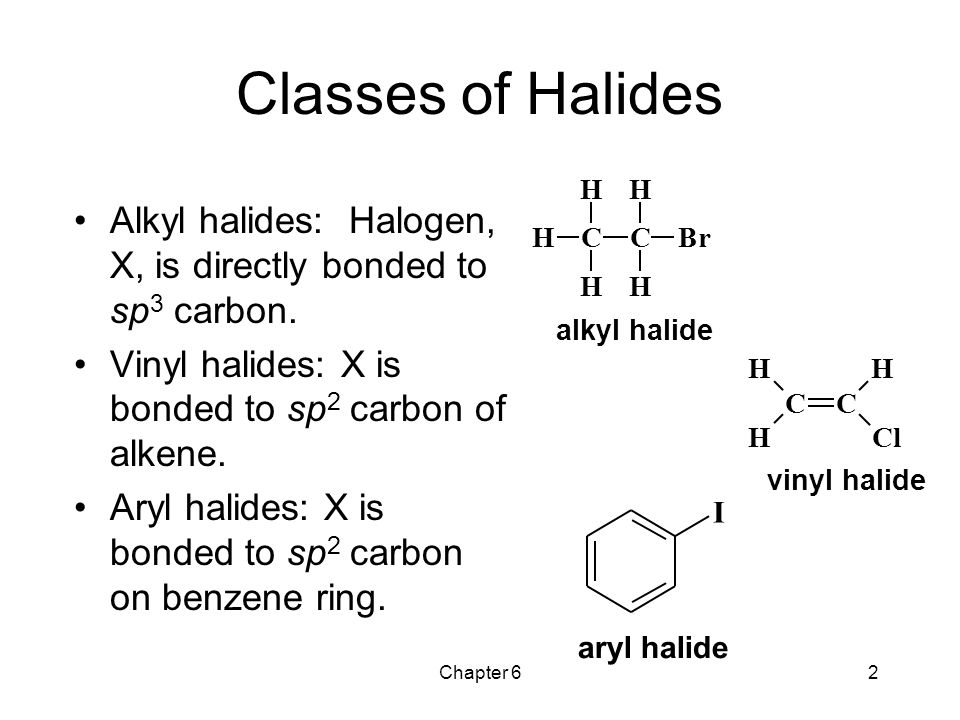
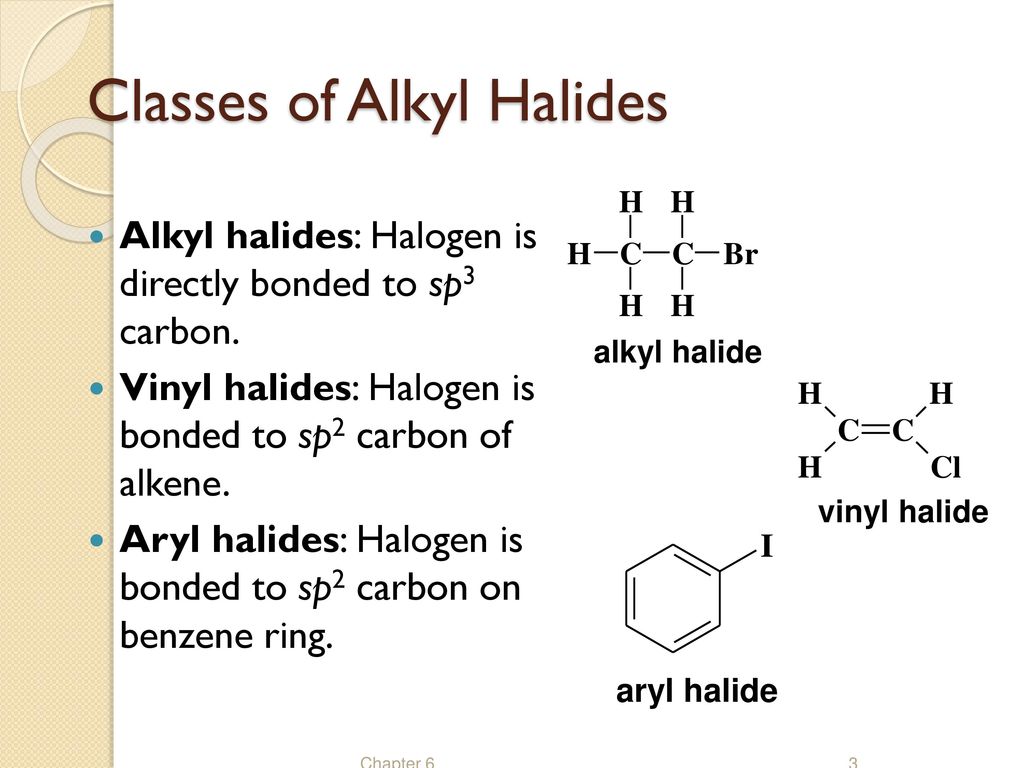
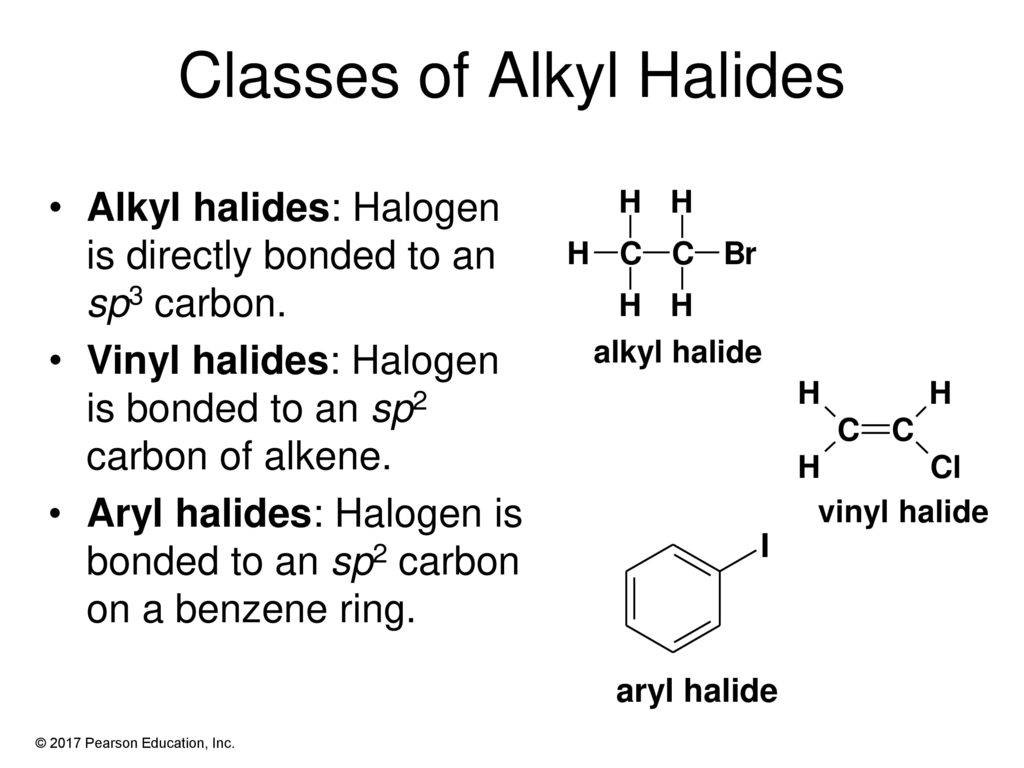
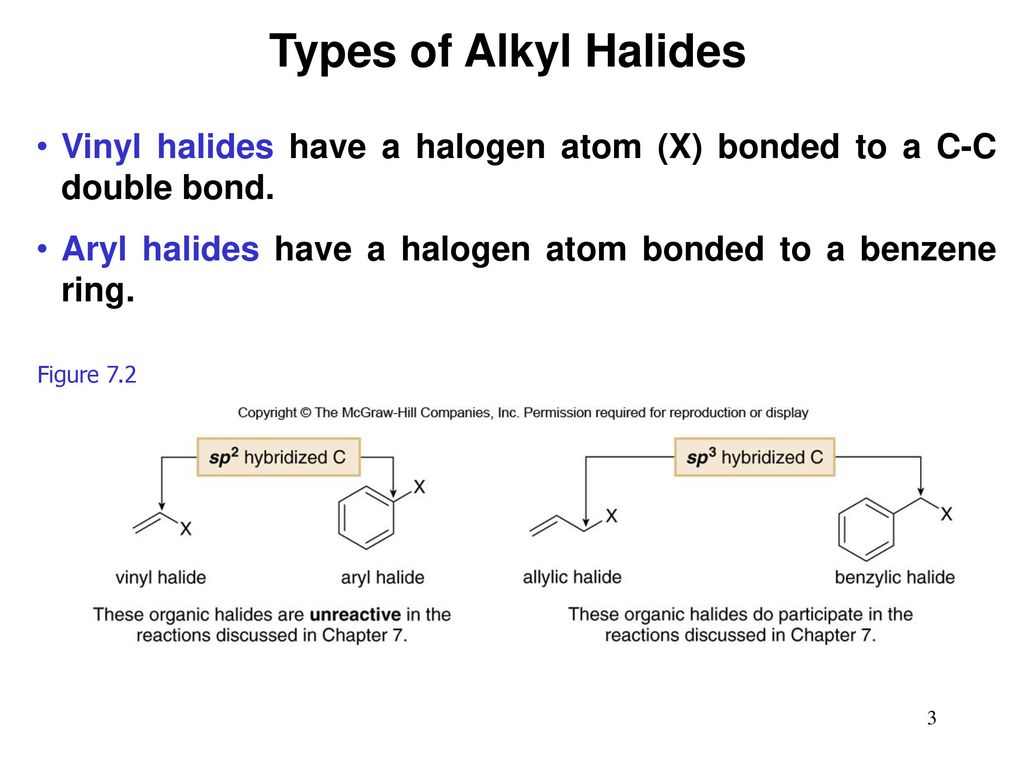
Alkyl vinyl aryl halides. There is an interaction between one of the lone pairs on the chlorine atom and the delocalized ring electrons and this strengthens the bond. Formally this is ethylene h 2c ch 2 with one of the hydrogens substituted by a heteroatom. For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides. A vinyl halide is clearly a species with a formula h 2c c x h in which a halide is directly bound to an olefinic bond.
The term vinyl is often used to describe any alkenyl group. Vinyl chloride h 2c ch cl is an example. S n 1 reactions are theoretically possible but not generally observed as the formation of the aryl cation is not energetically favourable. If the halogen atom halo group is bonded to a double bonded carbon a sp2 hybridized carbon atom then it is known as vinyl halide.
Step 1 of 3 if the halogen atom halo group is bonded to a single bonded carbon atom a sp3 hybridized carbon atom then it is known as an alkyl halide. The carbon chlorine bond in chlorobenzene is stronger than you might expect. Consequently this functional group is polarized so that the carbon is electrophilic and the halogen is nucleophilic as shown in the drawing below. The functional group of alkyl halides is a carbon halogen bond the common halogens being fluorine chlorine bromine and iodine.
The extra strength of the carbon halogen bond in aryl halides. An aryl halide has general formula c6h 5x in which an halide group x has substituted the aryl ring.