Acetic Acid Solubility In Water Vs Vinyl Acetate

Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light.
Acetic acid solubility in water vs vinyl acetate. Cas 108 05 4 ph 7 20 g l h o 20 c. Vinyl acetate is the acetate ester of vinyl alcohol. Acetic acid has been found in human liver and kidney tissues and has also been detected in most biofluids including feces urine breast milk and saliva. Upon treatment with a standard base it converts to metal acetate and water.
Vinyl acetate stabilised for synthesis. Ethyl acetate systematically ethyl ethanoate commonly abbreviated etoac etac or ea is the organic compound with the formula ch 3 coo ch 2 ch 3 simplified to c 4 h 8 o 2 this colorless liquid has a characteristic sweet smell similar to pear drops and is used in glues nail polish removers and in the decaffeination process of tea and coffee. Acetic acid is a drug which is used to treat infections in the ear canal. With strong bases e g organolithium reagents it can be doubly deprotonated to give lich 2 co 2 li.
Vinyl acetate c4h6o2 cid 7904 structure chemical names physical and chemical properties classification patents literature biological activities safety. This monomer is used principally in the production of polyvinyl acetate pvac and other vinyl acetate co polymers. Acetic acid exists as a liquid soluble in water and a weakly acidic compound based on its pka. Polyvinyl acetate is a precursor of polyvinilyc alcohol and polyvinyl acetate resins pva.
Vinyl acetate is a colorless flammable liquid that also has a characteristic smell that can quickly become irritating. Reduction of acetic. Reacts with air or water to produces peroxides that initiate explosively violent polymerization. Reacts with hydrogen peroxide to form explosive peracetic acid.
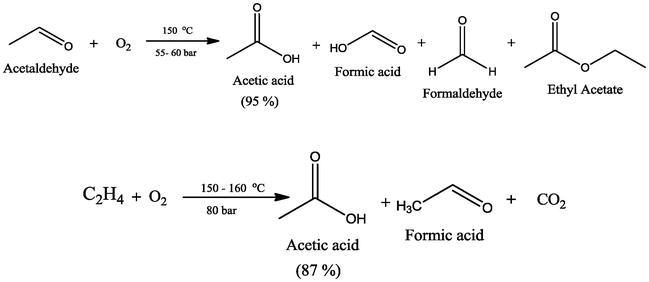
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst. Vinyl acetate is also copolymerized as a minor raw material for. Durability of concrete and cement composites 2007. Ethyl acetate is the ester of ethanol.
Since vinyl alcohol is highly unstable with respect to acetaldehyde the preparation of vinyl acetate is more complex than the synthesis of other acetate esters.