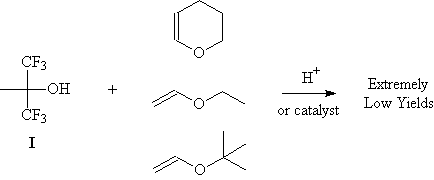
Acetal Vinyl Ether

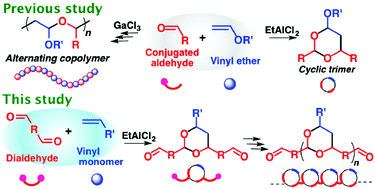
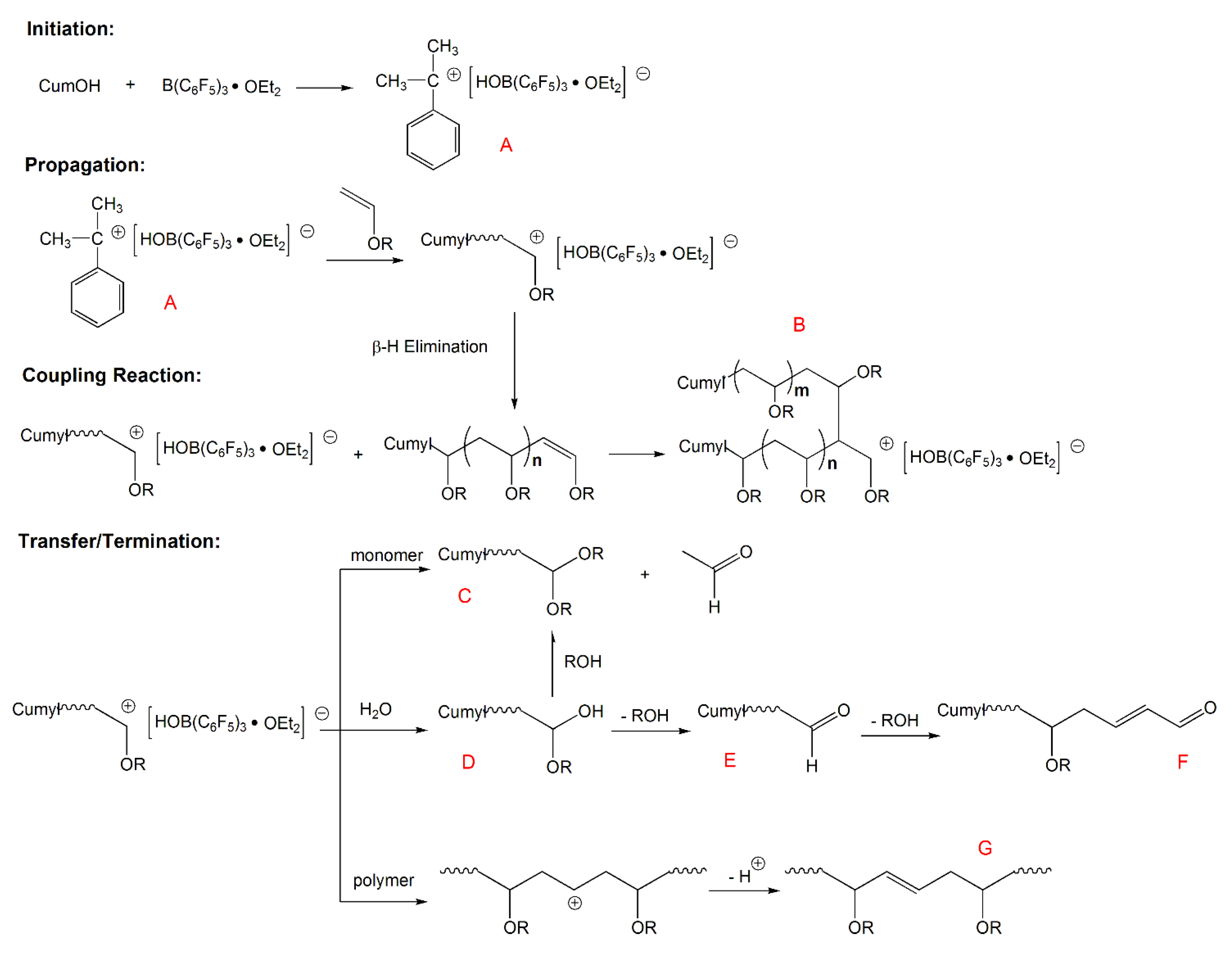
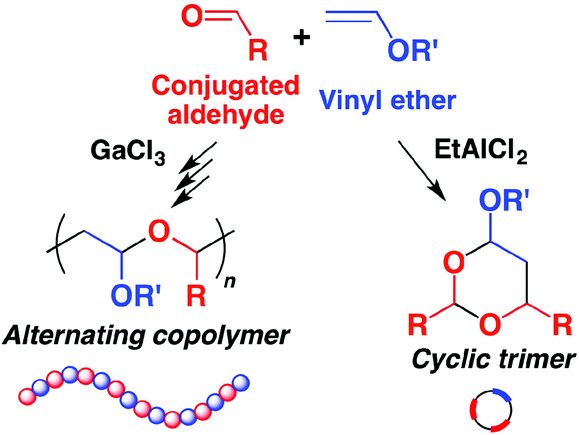
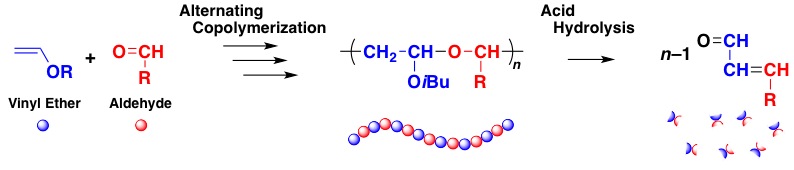
A polyaddition reaction via the cyclotrimerization of one vinyl ether and two conjugated dialdehyde molecules successfully proceeded using etalcl 2 as a lewis acid catalyst yielding a polymer with cyclic acetal structures in the main chain.
Acetal vinyl ether. Ethyl vinyl ether participates in many reactions of interest to organic synthesis. The acetal thermal decomposition decomposition system includes a gas phase system and a liquid phase system. The use of a low reactive or non polymerizable vinyl ether and the choice of adequate catalysts were extremely important for the effective. A series of aromatic acetals 1 from substituted phenols ch3ch oibu oar.
Hydrolysis of acetaldehyde diethyl acetal and ethyl vinyl ether. The vinyl carbonate 13 152 gives only copolymerization the ketene acetal 11 153 and the methyl vinyl ether 14 152 give both copolymerization and chain transfer in styrene polymerization whereas with the benzyl vinyl ethers 12 153 15 8 and 16 18 151 chain transfer is the only reaction detected. An alternative approach to the desired vinyl ether was inspired by the work of hoye and others who demonstrated the selective silylation ring opening of a 1 2 acetal to afford an allyl vinyl ether. Packaging 1 kg in glass bottle 100 500 g in glass bottle.
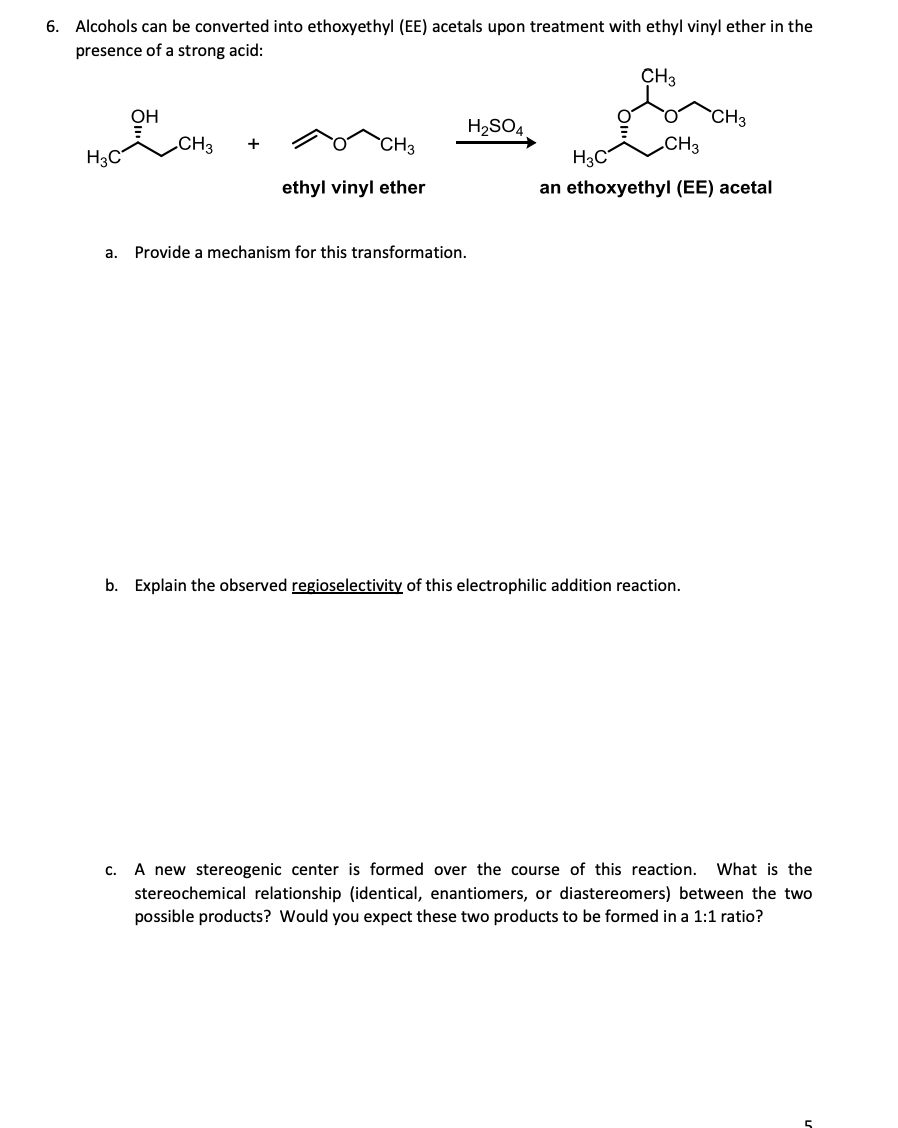
Furthermore the reaction enables a facile entry to labile diarylacetaldehydes by tfa mediated hydrolysis of the β β disubstituted vinyl ethers. Etoch ch 2 roh etoch or ch 3. With catalytic amounts of acids ethyl vinyl ether adds to alcohols to give the mixed acetal. Ar c6h5 p ch3oc o c6h4 p ch3c o c6h4 p no2c6h4 4 no2 2 6 di c6h5 c6h2 o ch3c o c6h4 were employed as new initiators in conjunction with lewis acids mtxn for the living cationic polymerization of isobutyl vinyl ether ibve.
This strategy was particularly attractive since it would avoid the use transition metal catalysis. This alcohol protection reaction is akin to the behavior of dihydropyran. Bromoacetaldehyde diethyl acetal has been used in the synthesis of monomer 2 2 2 dimethoxy ethoxyet hyl vinyl ether. It has been used as synthetic building block.
Chem 2013 78 9815 9821. The controlled cationic copolymerization of 2 chloroethyl vinyl ether ceve and various cyclic acetals successfully proceeded in a living manner. Importantly the copolymerization times 10 s 70 h and the sequences of the copolymers multiblock random or approximately alternating significantly differed depending on the cyclic acetal used. The gas phase cracking method uses a metal as a catalyst to cleave the acetal vapor in a high temperature environment and deposits a fine metal powder such as silver or gold on a carrier as a catalyst and cleaves the acetal at 25 c to 40 c to form an olefin ether.